Quality Tolerance Limits and Quality Metrics Consulting Support
 Article: ICH Guidelines & Industry Inefficiency
Article: ICH Guidelines & Industry Inefficiency Article: Taking Control Of QTLs In Clinical Trials
Article: Taking Control Of QTLs In Clinical Trials Are you prepared for an inspection?
Are you prepared for an inspection? Blog: Importance of QTLs
Blog: Importance of QTLs Frequently Asked Questions
Frequently Asked Questions Printable PDF on QTLs
Printable PDF on QTLs Quality Management Systems
Quality Management Systems Webinar: QTLs as required by ICH E6 (R2)
Webinar: QTLs as required by ICH E6 (R2) Webinar: Risk-Based Monitoring Post-(R2)
Webinar: Risk-Based Monitoring Post-(R2) Have questions? Contact Us
Have questions? Contact Us
Avoca’s Consulting Service for Quality Tolerance Limits (QTLs) aims to help sponsors and CROs comply with ICH E6 (R2) by providing study teams with training and support for implementing QTLs.
Why are Quality Tolerance Limits Important?
QTL parameters are absolutely critical to basic trial integrity, patient safety, and the primary endpoint. The goal of a QTL is to detect systemic risks – those that will affect the study and make it very hard to draw the right conclusions from your results. Examples include inclusion/exclusion protocol violations, incomplete/missing endpoint data, and AEs/SAEs of special interest. QTLs need to be proactively defined at the planning level of the trial in coordination with risk assessment activities.
Learn how QTLs fit within an overall quality management system (QMS) ›
The Avoca Advantage
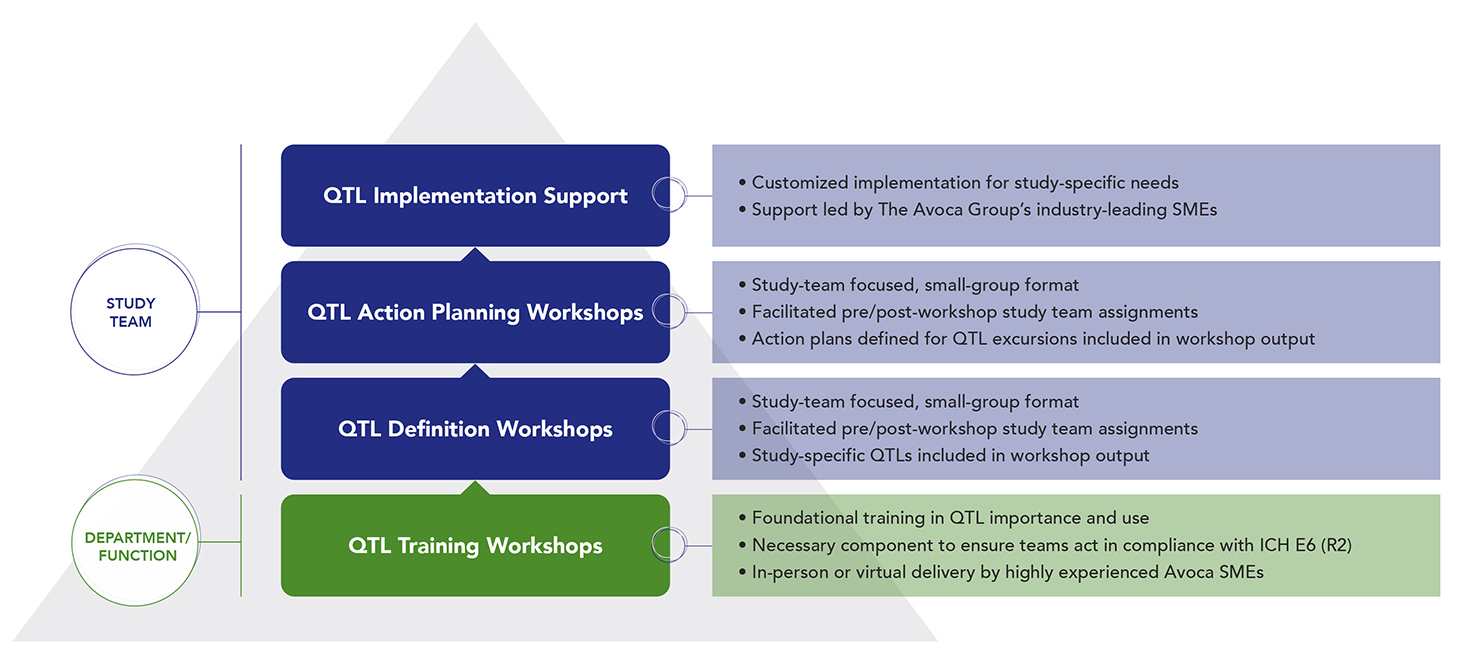
This service is based on our 20+ years of experience in clinical quality improvement – helping sponsors and CROs to measure, manage, and improve quality in clinical trial execution. Our subject matter experts (SMEs) provide comprehensive support for planning and implementing QTLs for ICH E6 (R2) compliance, either as individualized QTL support or as part of overall Quality Management System (QMS) implementations:
QTL Implementation Support | QTL Action Planning Workshops | QTL Definition Workshops | QTL Training