Opportunities and Risks Abound in Transition to Patient-Centric Research Models
Written and submitted by Craig Morgan, Head of Marketing, Study Startup, Oracle Health Sciences – an AQC Member company.
Very little has changed in traditional clinical trials since their inception to ensure the efficacy and safety of drugs in human subjects. Borne out of academic medical centers they have expanded to include community hospitals, free-standing research sites, and physicians in private practice becoming involved as research sites. However, a confluence of factors is forcing many in the industry to “re-think” this approach to traditional studies, as stakeholders want to rein in drug development costs and speed therapies to patients.
The traditional approach to clinical research is buckling under pressure from competition to sites and a patient population not able or willing to take part in studies, as the number of studies initiated continues to grow. More than 80% of clinical trials fail to meet original timelines, with enrollment problems largely to blame, branding the site selection process a perpetual bottleneck. According to research from the Tufts Center for the Study of Drug Development (CSDD), 37% of sites under-enroll and 11% never enroll a single subject. And while 89% of studies do eventually meet enrollment targets, sponsors often get there by doubling their original timelines or engaging in the costly practice of adding rescue studies, due to poor site selection and under-enrollment. In a typical Phase III study, this can translate into $2.25 million in expenses for non-active and under-enrolling sites. Overall, choosing the wrong sites can boost the cost of trials by 20% or more.
Moreover, recognizing the opportunity to engage with highly motivated patients or patient organizations, to both reduce costs and improve success in clinical trials, pharma companies are beginning to explore the concepts of patient-centric and patient-led trials.
The idea of “virtual” or “decentralized” clinical trials is becoming an actively discussed alternative, although other terms are commonly used to refer to this transition along a continuum from traditional to hybrid studies, such as “digital trials”, “siteless trials”, etc. But regardless of modality all studies are utilizing technology as an enabler, and all studies must be conducted in accordance with regulatory standards.
Decentralized clinical trials offer a more patient-centric approach in which fewer clinic visits are required and patient and caregiver burden are reduced. Decentralized clinical trials utilize a wide range of digital technologies to collect safety and efficacy data from study participants, normally remotely from the patient’s own home.
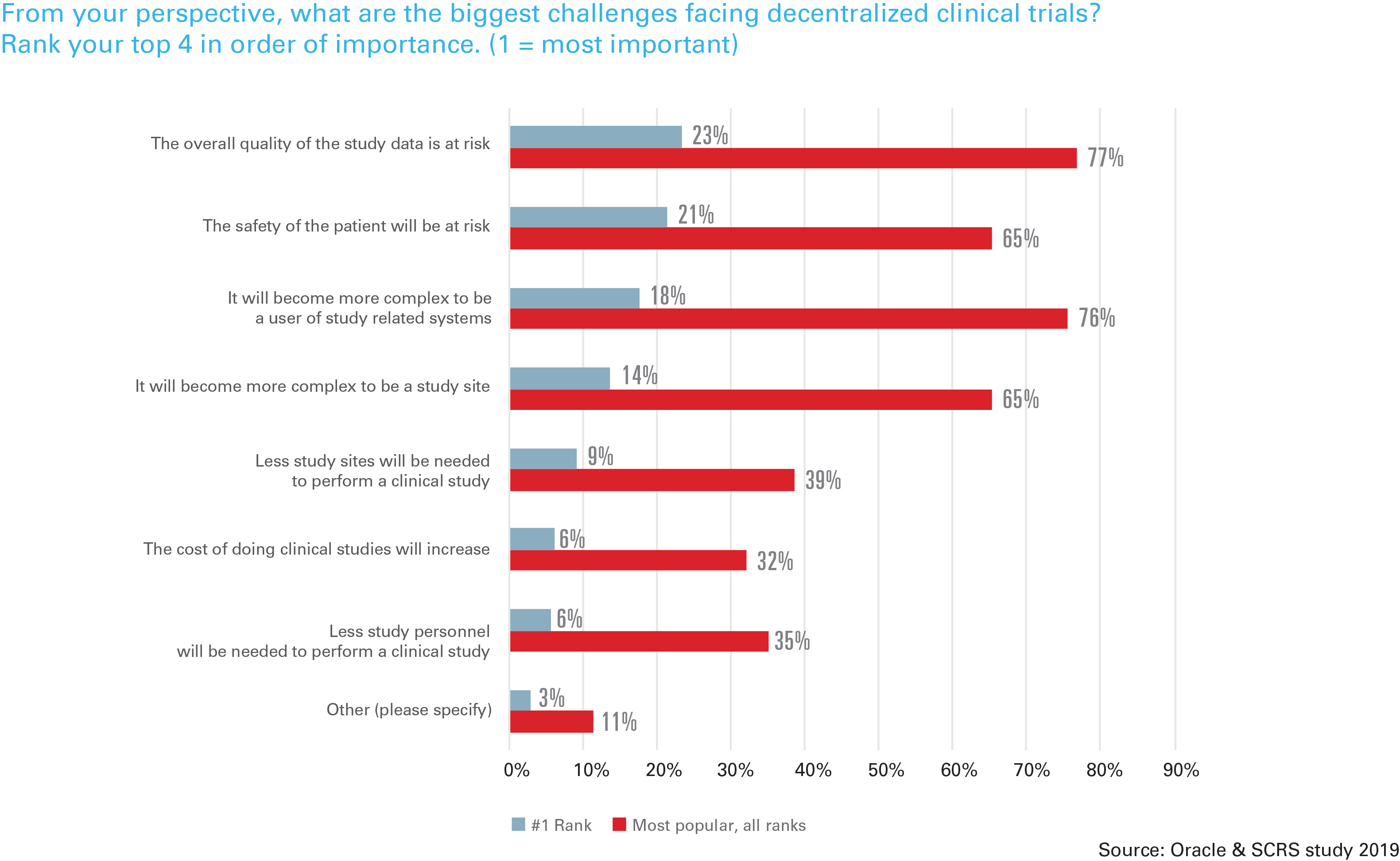
With any change comes opportunities, uncertainty and challenges. A recent comprehensive study conducted by Society for Clinical Research Sites (SCRS), Impact Assessment of eClinical Technologies and Industry Initiatives on Sites, sought to understand the impact of patient-centric study design, such as decentralized clinical trials on investigative sites.
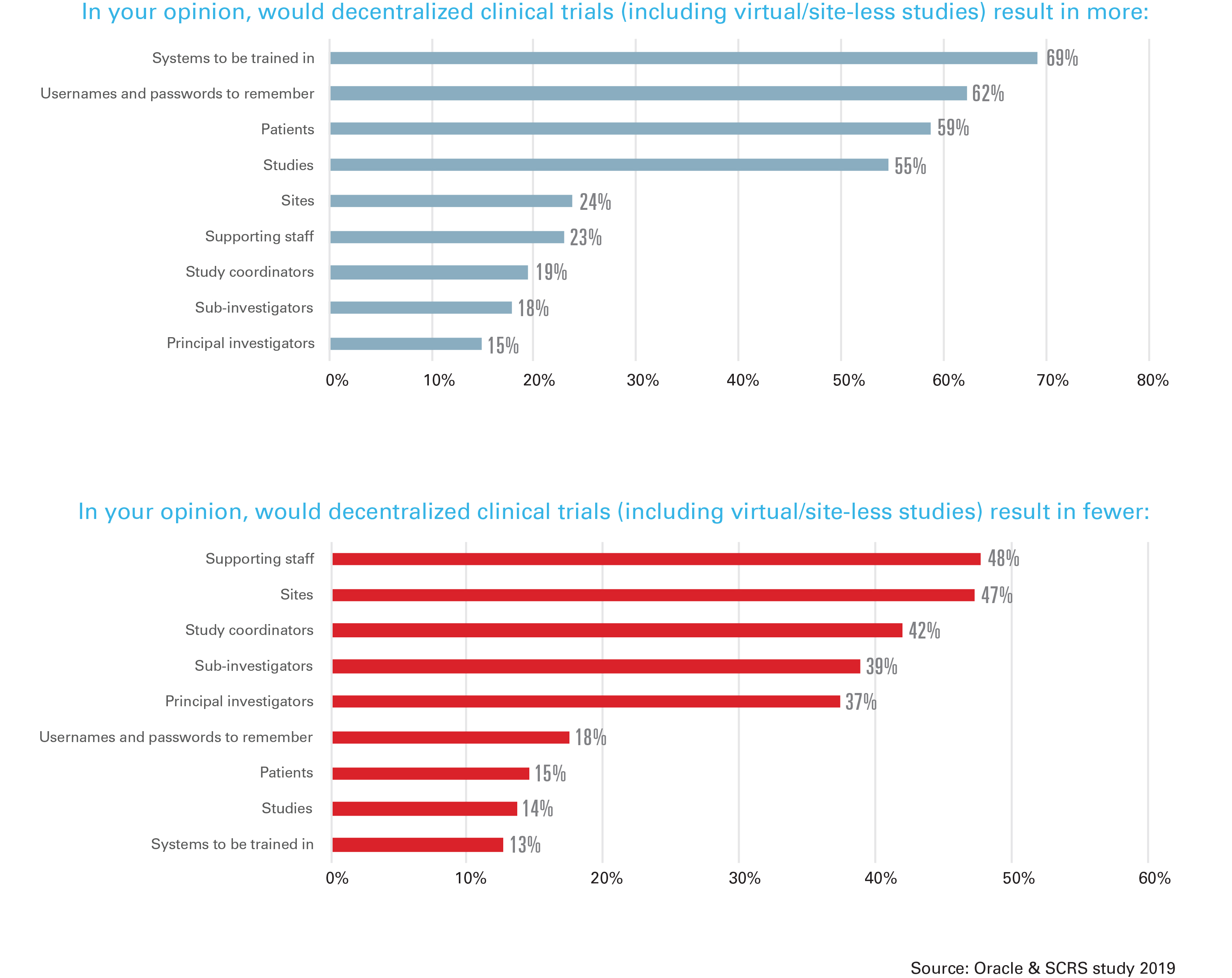
While respondents cited greater patient participation (82%) and patient access (71%) as positive anticipated benefits, they cited more systems to be trained in (69%), more usernames and passwords (62%), and fewer sites (47%) and staff (48%) as negative anticipated results. In addition, respondents expressed concern about the overall study data quality with a decentralized model, and 65% worried that the patient’s safety would be at risk with a decentralized model. Distance to a study site and the frequency of required visits was mentioned by all respondents as an enrollment challenge.
Today, a plethora of technologies exist to assist in the execution of patient-centric studies, but these technologies are only part of the solution. A holistic approach is required to ensure that GCP guidelines and regulatory standards are adhered to, and that challenges associated with this new paradigm of conducting clinical research, including ethical challenges, are identified and addressed as more clinicians move toward this model of research. Imperative in this transition is the on-going discussions amongst industry groups and stakeholders.
Additional Resources:
Webinar: Will Decentralized Clinical Trials Compound Patient and Data Quality Risks?
Presented October 29, 2019
This webcast focuses on understanding risks incurred by adopting the technical infrastructure required for decentralized clinical trials and how they can be mitigated.
