SPONSORSHIP
Why Sponsor?
Since the Avoca Quality Consortium (AQC) launched seven years ago, its mission and purpose has been to transform clinical trial execution through a greater alignment between key stakeholder groups and to move the needle on quality in clinical trial execution. Today, as one of the earliest consortia in the clinical trial space, the AQC has grown to 80+ Member companies, which include pharmaceutical/biotech firms, CROs, and specialty service providers.
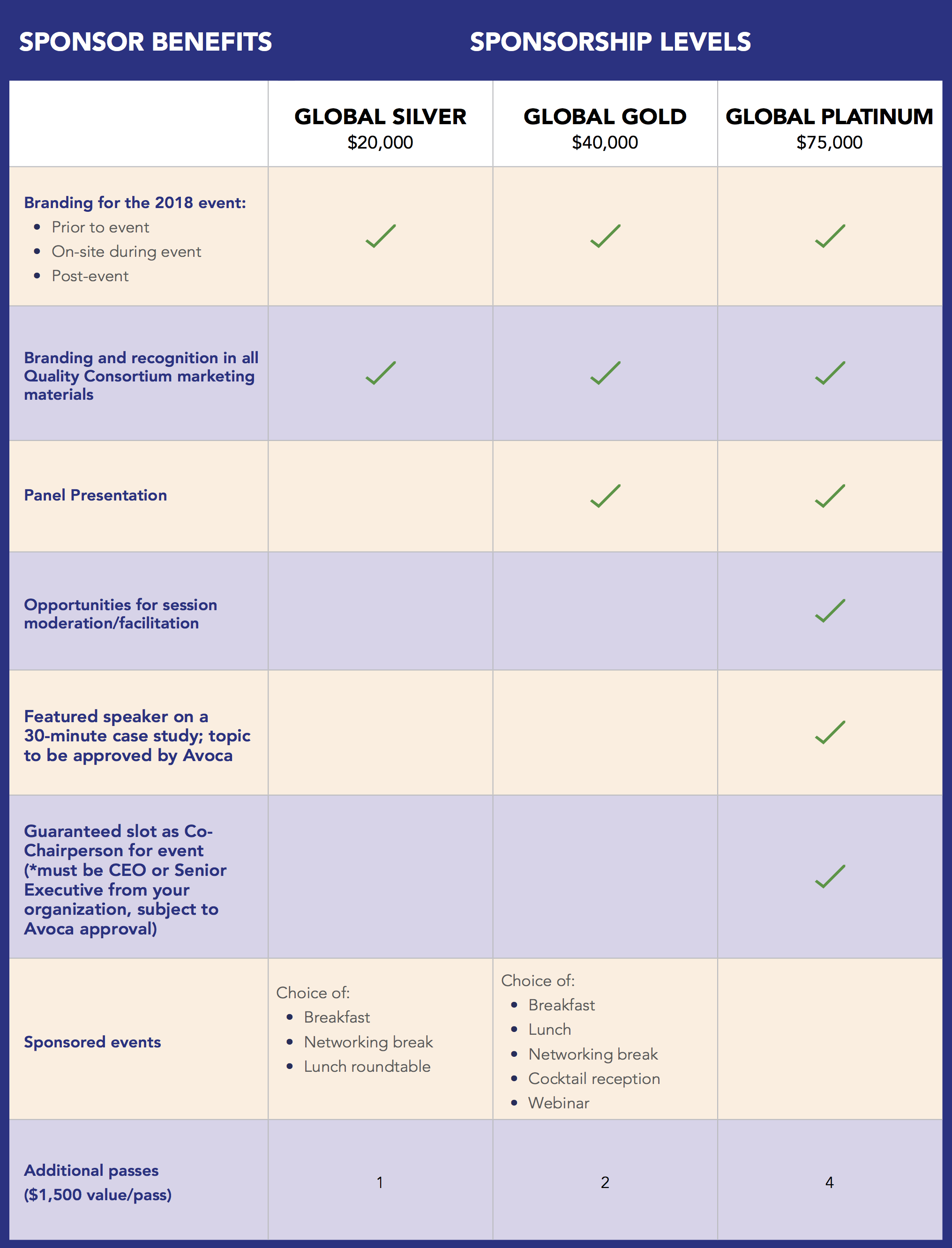
To further the mission of the AQC, our annual Global Summit captures what’s top of mind for leaders and helps attendees understand how clinical trial execution will evolve in the future. Sponsorships maximize your exposure to top industry decision-makers before, during, and after the event through a variety of packages which include speaking sessions, prominent branding, targeted email blasts, and webinar participation, reaching key life science industry professionals from our extensive AQC database. And, it helps to support the AQC mission to drive efficiency, improve quality, and mitigate risk in clinical trial execution.
See What Industry Leaders Are Saying
“This year’s Summit has been even better than the one I attended last year, and that was really difficult because last year was already great. I’m amazed how things had advanced since last year. The AQC is really delivering very interesting tools and approaches and promoting real and effective collaboration for being better and faster, indeed.”
“There has been a lot of groundbreaking activity with the Consortium among the companies that are participating in really understanding that the quality process is one where you don’t want to be different than everybody else. You benefit from standardization and that allows you to work the same way with different providers, and Avoca has helped to illuminate that. We have put in place a lot of standards that companies are using uniformly across the industry and that reduces variability and enhances quality and ultimately compliance.”
“The Summit brings us together as an industry, whether we are a Tech provider, a CRO, a Sponsor, a big Pharma, or a Biotech. We are all dealing in the same ecosystem, so it really does allow us to come all together and share those experiences and learn from each other.”
“We have addressed multiple items that were challenges for us as a CRO with our clients and sponsors across the industry: Quality Management, System Framework, Quality Agreements, Vendor Due Diligence, Inspection Readiness. These are all things that have been a great benefit. To add to that, it’s a huge forum for collaboration and networking.”
Key Deadlines
Deadline for sponsorship agreements: 15 May 2018
For more information or to become a sponsor, contact Caryn.Laermer@theavocagroup.com