Oversight Capability Maturity Model®
The Avoca Quality Consortium (AQC) has developed an Oversight Capability Maturity Model (OCMM) to assess organizational capabilities for oversight of external service providers in both regulated and non-regulated scenarios. A first-of-its-kind for the Pharmaceutical R&D industry, the OCMM® enables organizations to manage internal capabilities for provider oversight and identify weaknesses. This process will help organizations manage providers’ ability to deliver on time, on budget and with expected quality, which is increasingly important to ensure ICH E6 (R2) compliance.
The OCMM has five levels of maturity, and addresses ten critical dimensions for Oversight Capability. Each dimension is linked to a wide range of AQC leading practice guides, tools and templates. For example, one of the dimensions of oversight capability is governance. Many organizations struggle with developing effective governance constructs such as committee charters. Having governance charters in place helps to drive effective joint collaboration to better ensure successful delivery on the goals and objectives of the partnership. The model links users to sample governance committee charters, such as an Executive Governance Committee Charter.
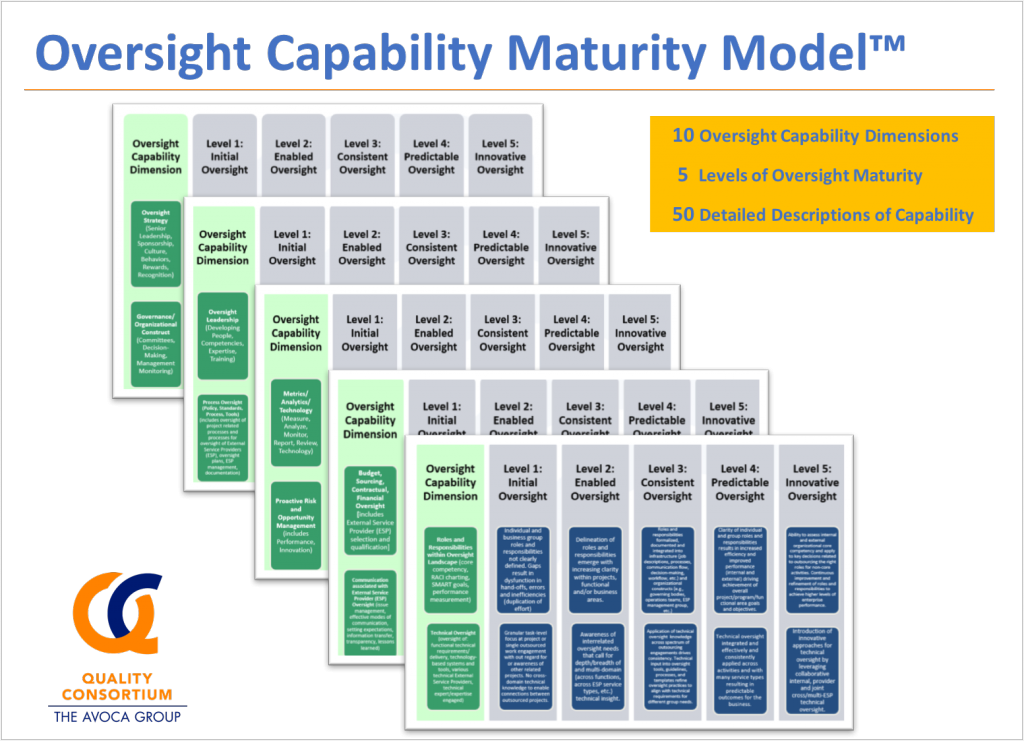
The outcome of this OCMM is a matrix with 50 detailed descriptions of oversight capability (5 levels and 10 dimensions) that can be used to assess your organizational maturity level and to advance it to higher levels.
About the Avoca Quality Consortium
|
|

