Risk-Based Quality Management: Working It Out in the Trenches
With the adoption of ICH E6 (R2), risk-based quality management for clinical trials has become an expectation across the pharmaceutical industry. So, how is it working out in the trenches of daily operations? The Avoca Group’s research, documented in the 2017 Avoca Industry Report, indicates that, while there are concerns, there is also potential for great value.
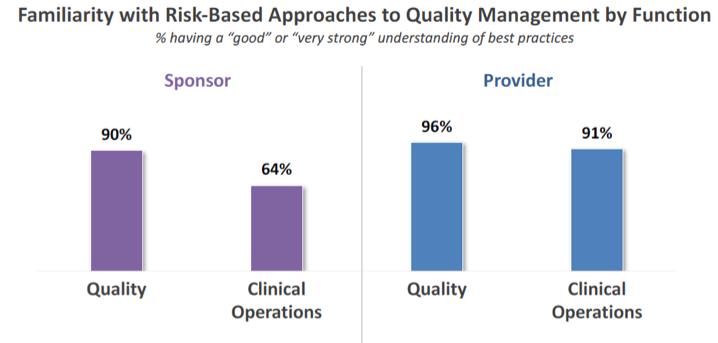
To begin, it is important to establish how familiar sponsors and providers are with risk-based approaches to quality management. Avoca’s research reveals that nearly 70% of sponsor respondents indicate having a “good” or “very good” understanding of best practices in risk-based quality management. While this is encouraging, it is significantly lower than the nearly 90% of providers who indicate this level of knowledge.
Breaking this down further, among providers, understanding of risk-based quality management was consistent by function across Quality and Clinical Operations. However, among sponsors, those in Clinical Operations expressed a substantially lower level of familiarity than did those representing the Quality function.
We can view this gap as an opportunity for Quality and Clinical Operations to increase collaboration and knowledge sharing. It may also indicate the need for a mindset shift in sponsor organizations from viewing quality management as “Quality’s job” to seeing it as “everyone’s responsibility.”
Familiarity with risk-based quality management protocols will also undoubtedly improve as the frequency of use increases. Currently, approximately 50% – 60% of sponsors and providers reported that risk-based quality management is being utilized in at least half of the trials they run. That is a positive sign but leaves significant room for growth.
Utilization is one thing – results are another. The Avoca report notes that approximately half of respondents indicate that risk-based quality management has been “very” or “extremely” impactful on increasing quality in trials; far fewer, however, feel this is resulting in improvements to efficiency in terms of time or resources.
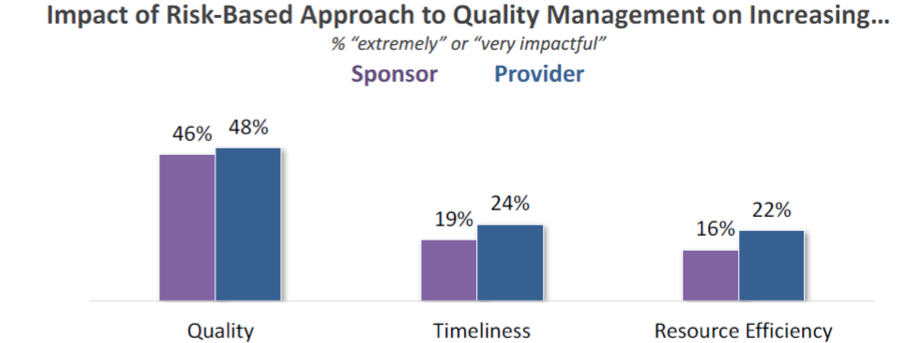
When we put all these statistics together, what do we see with regard to risk-based quality management?
- Familiarity is good overall … but it could improve.
- Utilization is reasonably good … but it could improve.
- Impact is trending in a good direction … but it could improve.
This assessment might leave you feeling ambivalent about the long-term effectiveness and sustainability of risk-based quality management. But numbers aren’t the only considerations. The 2017 Avoca Industry Report also gathered verbatim commentary from respondents on the use of risk-based quality management. That commentary suggests that the use of risk-based quality management can positively influence awareness, communication, and strategy within and between sponsor and provider organizations. For example, respondents noted:
- “Planning has opened communication amongst team.”
- “Increased awareness of threats to quality of study.”
- “Our teams are constantly meeting and reassessing to make sure that the potential risks and actual risks are being pointed out and dealt with.”
Certainly, challenges were also reported, such as gaining alignment on rationale, process, and measurement. But even these challenges have their positive aspect if they drive greater collaboration between Quality and Clinical Operations, and among sponsors and providers.
Taken all in all, if risk-based quality management is bringing people together in new ways, that can only be viewed as valuable to the future of clinical trials and to the patients who are ultimately being served.
Are you concerned about compliance and effectiveness of your QMS or risk-based oversight practices? Avoca can help. We specialize in installing or remodeling Quality Management Frameworks that comply with ICH E6 (R2) and utilize risk-based approaches to quality and oversight. Avoca’s consulting services are grounded in the leading practices and tools from the Avoca Quality Consortium® and customized to drive measurable improvements to quality and execution of clinical trials. To receive information or to speak with a representative, contact us.
Download The 2017 Avoca Industry Report: Using Risk-Based Approaches to Quality Management
.
Author:
 |
Dennis Salotti
Vice President, Operations, The Avoca Group |

