You Can’t Manage What You Don’t Measure
Risk is our constant companion. As clinical trials grow in complexity, so do risk-based challenges associated with bringing new therapies to market. The process of initiating clinical trials, collectively known as study startup, is cumbersome, challenging and fraught with delays. Study startup has an impact on patient recruitment, trial duration and associated costs, and unfortunately, it is the worst performing stage of any study.
Where are the bottlenecks? Where are studies most at risk of missing timelines? Obvious questions to ask but difficult to answer using conventional tools, which lack the ability to track activities at a granular level. Manually prepared data are often too old to reliably convey study startup status, causing executives to demand faster and greater visibility into data generated by study startup activities. Existing processes and procedures often lead to a false sense of security, which results in decision making paralysis, rather than enhancing productivity and promoting efficient business operations.
There are numerous steps involved in starting clinical trials, and without risk management planning, each has potential for causing delays, and ultimately, jeopardizing the study. To mitigate this situation, technology providing risk management capabilities is a critical improvement over Excel, the incumbent tool used to track study initiation processes. A major shortcoming of Excel is its lack of project- and risk-management functionality.
A practical risk management methodology enables stakeholders to identify clinical trial issues early on, which in turn allows for the design of effective mitigation strategies before risks materialize. Risk mitigation is therefore optimal using systems which can provide timely, preferably real-time data on trial bottlenecks, which indicate red flags to be reviewed and addressed or at least tracked carefully throughout the trial.
For organizations looking to increase transparency in the study startup process, the sheer volume of data and the functional silos in which they exist can be a daunting hurdle. New generation systems enable teams to capture, analyze, share and visualize study startup data in one system. With the help of leading practice guides, tools, templates, and automated workflows for study startup, Sponsors and CROs can proactively conduct risk management.
ICH E6 (R2) guidelines state that risk-based methodologies should begin at the start of a trial. To conform to these regulatory guidance’s for identifying and mitigating risk, forward thinking industry leaders have been trading in their Excel spreadsheets in favor of custom-build study startup applications.
As Crissy MacDonald, Executive Director, Client Delivery, The Avoca Group explains, “ICH E6 (R2) requires risk-based methodologies be incorporated into clinical trial execution. The best way to implement risk-based methodologies is to do so with data whenever possible. By utilizing a data driven approach to risk-based decision making, you are focusing in on the key areas of risk and are more effectively able to manage and mitigate those risks as the parameters being followed reach a potential point of action.”
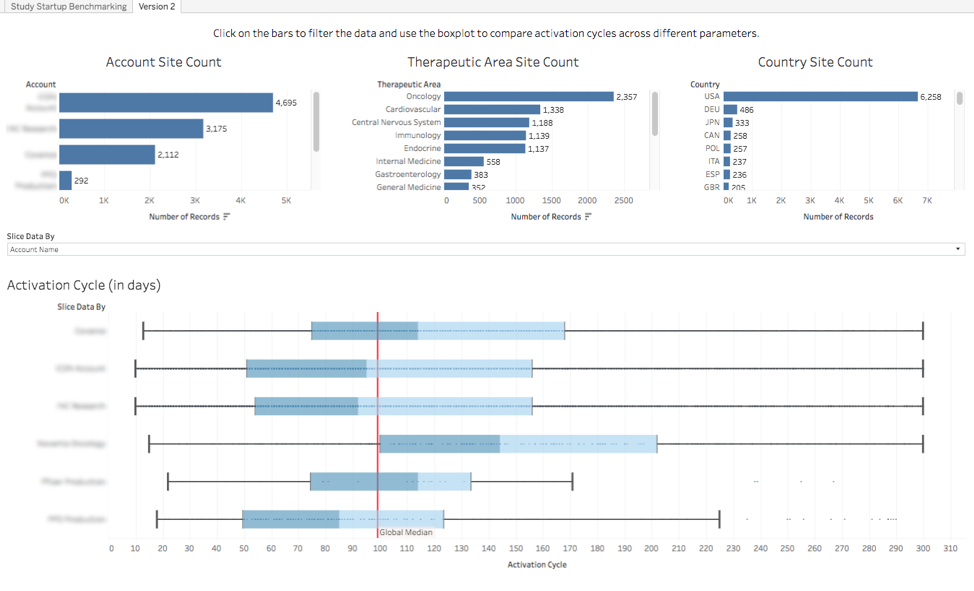
Fig 1: Benchmarking report of study activation cycle times across therapeutic areas
To support compliance with ICH E6 (R2) and application of risk-based approaches for study startup (such as regulatory submissions), sponsors should consider applying a quality-by-design approach. To do this, teams should identify factors that are critical to quality (CTQ), assess and prioritize those factors, and then develop plans for risk control (define tolerance limits, mitigation plans for prevention and contingency plans to trigger if the risk occurs).
Trial project managers can know exactly if a study is on track and if CTQ factors are outside tolerance limits not, take the required actions to remove bottlenecks and eliminate or refine activities to ensure that regulatory submissions are ready by the submission dates. Having access to the data trends in a central location drives decision-making, allowing study teams to focus on potential risks and the most critical data and processes necessary to achieve study objectives–a risk-based, data-driven approach to study startup.
What does it take to implement a risk-based approach to study startup?
- Establish Data Transparency: Increasing transparency enables the identification of key trends and processes.
- Promote Collaboration: Globally dispersed, cross-functional teams need a way to collaborate in real-time and track study startup progress.
- Identify and Optimize Key Processes: Real-time metrics generated as the study proceeds provide visibility into processes, indicating those ripe for optimization or risk tracking.
- Monitor Progress: Data visualization with predictive trend monitoring throughout the study empowers proactive risk management via comparison with internal/external benchmarks.
- Lifecycle Optimization: A holistic view of the processes and how they may impact downstream activities should be utilized, critical to ensuring quality and regulatory/SOP compliance.
Download recorded Webinar and learn how to deploy an effective risk management approach to study startup.
Presented by The Avoca Quality Consortium and goBalto on October 9, 2018.

