Technology and Risk: How Systems and Software Are Impacting ICH E6 (R2)
Sponsors and providers alike in the pharmaceutical industry are being confronted with the need to leverage technology to meet the demands of the risk-based ICH E6 (R2) guidelines. The reason is simple: the guidelines have shifted the focus of inspections into new territory, and existing legacy systems and disparate data sources can’t cope with the new demands being placed on them. The challenge, however, is that the technology that is needed is not yet mature in all cases.
The Avoca Group’s research, documented in the 2017 Avoca Industry Report, provides key insights into the complex dance between technology and risk.
Who is adopting technology for risk-based approaches?
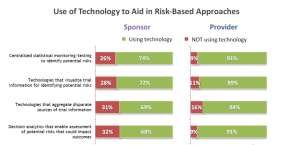
To begin, there is a notable difference in technology adoption rates between sponsors and providers, with providers showing a higher adoption rate across the range of technologies evaluated (Figure 1). This is understandable, since providers are higher utilizers of technology in the course of their work. It is in their best interest to leverage any tool with the potential to speed up efficiency and improve decision making.
Figure 1
Exactly what is being adopted?
Figure 1 shows that, among Sponsors, there is a slightly higher utilization of less advanced technologies, such as statistical monitoring and data visualization, than there is of more advanced technologies, particularly decision analytics. This is important to note, as machine learning (ML) and other forms of artificial intelligence (AI) involved in decision analytics are what will identify patterns of potential risk over the course of multiple clinical trials and help Sponsors align with ICH E6 (R2) guidelines.
How is technology working out in managing risk?
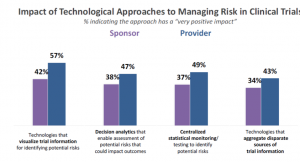
The answer to this question is both good and bad. Approximately half of providers say the approaches are having a “very positive” impact (Figure 2). Sponsors were somewhat weaker in their response, which may be due to the fact that they are in earlier stages of adoption of the technologies (Figures 1 & 2). That being said, a significant proportion of both sponsors and providers do not see technology as having a “very positive” impact on managing risk.
Figure 2
Why isn’t there a greater positive impact?
The technologies that are available to support risk-based approaches to clinical trials are not yet mature. This is still a nascent technology environment with a great deal of room for improvement. Technology providers have a tremendous opportunity here to create or refine solutions to deliver a compelling user experience and outstanding return on investment.
Where is the “sweet spot” for technology in a risk-based environment today?
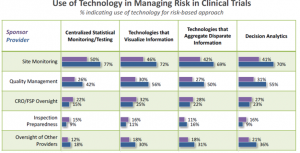
While, overall, technology has room for improvement to support risk-based management, there is a sweet spot that sponsors and providers can – and are – taking advantage of right now. That is in the area of site monitoring (Figure 3). Quality management is also being leveraged, to a slightly lesser degree.
Figure 3
Where could technology become transformative for risk-based management?
Looking at Figure 3, technology providers could transform clinical trial management by developing ways to improve provider oversight and inspection preparedness. These will be challenging, since the objectives and direct measures of success are arguably less discrete than for tasks such as site monitoring and quality management.
How happy are sponsors and providers with the technology they are using?
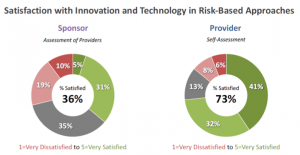
Both sponsors and providers are employing technology in risk-based approaches (Figure 1). But how happy are they about it? Here, we see a huge gap. Almost three-quarters of providers are satisfied with how technology is supporting risk-based approaches to clinical trial management, whereas only 36 percent of sponsors report a similar level of satisfaction (Figure 4). Considering the degree of perceived impact reported in Figure 2, it is understandable that sponsors have a low satisfaction level. Providers appear to be more generous with their assessment.
Figure 4
Where do we go from here?
Innovation demands change, and change is always uncomfortable. However, in the case of ICH E6 (R2), such change is demanded from both sponsors and providers. Technology is making headway into the risk-based environment, but technology providers have further work to do to prove the value of their tools and to help sponsors and providers become confident using the tools. The technology providers who do so will become key partners in transforming the risk-based landscape.
Are you struggling to determine how to identify, qualify, and select the right technology to support risk-based approaches at your company? The Avoca Group specializes in creating and improving ICH E6 (R2)-compliant Quality Management Systems and provides guidance in assessing, selecting, and implementing technology to support risk-based approaches to quality and oversight. The Avoca Group’s consulting services are grounded in the leading practices and tools from the Avoca Quality Consortium® and customized to drive measurable improvements to quality and execution of clinical trials. To learn more, or to speak with a representative, contact us.
Download The 2017 Avoca Industry Report: Using Technology in a Risk-Based Environment
.
Author:
 |
Dennis Salotti
Vice President, Operations, The Avoca Group |

