Recognizing and Addressing Inefficiencies in Vendor Qualification – One Step at a Time
With the continued commitment to clinical outsourcing by sponsors to achieve their goals comes a need to improve our working relationships. This has become particularly important due to the requirement for sponsors to improve provider oversight to comply with ICH E6 (R2).
To begin, we can focus on the vendor selection and qualification process, which is the starting point for any sponsor/provider relationship. At The Avoca Group, we are particularly interested in this process. We continuously hear from sponsors and providers alike that it is rooted in redundant tasks – sponsors repeatedly qualifying and selecting providers for each new clinical trial and providers responding to similar requests in slightly different ways, maintaining different document sets for each. And, because of these inefficiencies, we unnecessarily tie up resources, while lacking clear risk mitigation or improvements in quality and service delivery.
An important first step toward overcoming our inefficiencies in vendor qualification and selection is reaching a common understanding of expectations regarding roles and responsibilities throughout the entire clinical trial, which can be facilitated through the establishment and use of industry standards. Based on research that we conducted in 2013 at the request of Avoca Quality Consortium® (AQC) Members, we finally started to understand that there were resource limitations on both sides, a lack of internal expertise for the activities being conducted, and an inability to easily access the required documents to evaluate a provider.
Recognizing that sponsors/CROs and providers alike were interested in industry standards and industry-wide centralization supporting the qualification process, we initiated the long, arduous process of developing industry-leading standards for providers, followed by core and category-specific RFI questionnaire templates. In the pilot phase with our AQC Members, we found inconsistent use of tools that were meant to drive consistency and robust, high quality data for qualification decisions, and the Member companies reported wanting a centralized system for qualification and selection decisions.
The Diligent® Qualification Platform was born – a centralized, searchable repository of completed RFI questionnaires. During the proof of concept in 2016-17, agreements, contracts, and infrastructure were developed. Thirteen sponsors and 50 providers participated, resulting in more than 100 completed RFIs and over 200 RFI requests through the centralized model. We refined the system using feedback from participants and prepared it for widescale use.
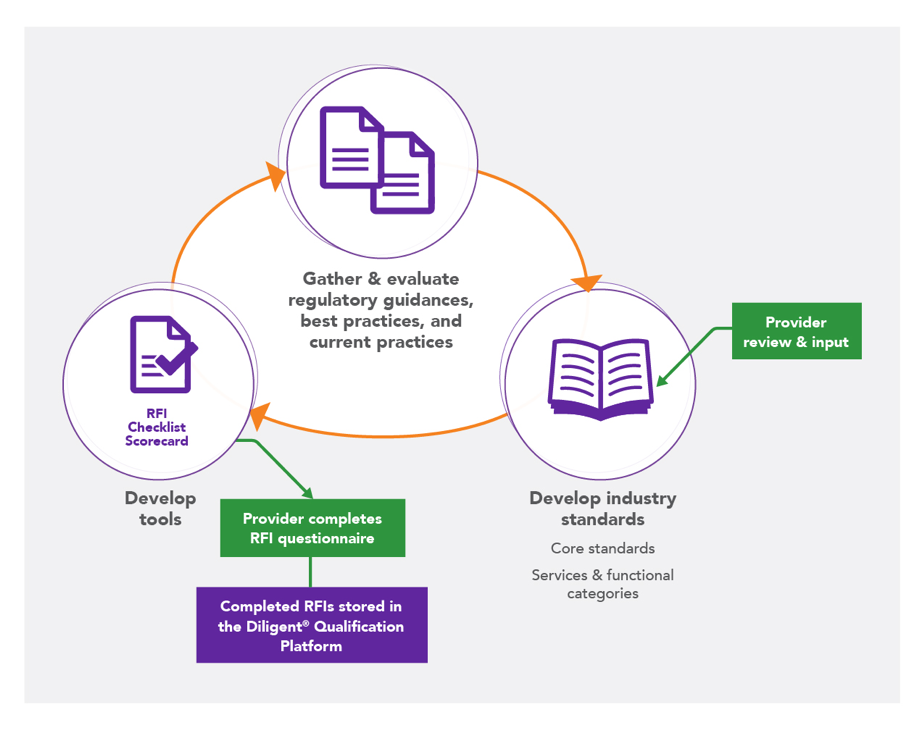
The AQC develops and maintains qualification-related tools based on industry standards; these tools are used to support vendor qualification decisions
We’re excited about the possibilities of this fit-for-purpose technology – a knowledge base that can support qualification; central repository of information based on the only industry-accepted standard for clinical services; real-time updates of information by sponsors and providers; and search capabilities to find the right provider to fit each trial. The standards on which the questionnaires are based reduce potential risk to quality and compliance, both increasingly important topics.
We continue to develop and refine the system, as more companies begin to use it and as we complement it with our consulting services. To learn more about the process and outcomes related to vendor qualification and the Diligent Qualification Platform, read the full-length article on this topic in Life Science Leader. To learn more, or to speak with a representative, contact us.
May 1, 2018 Clinical Leader Article:
.
Author:
 |
Dennis Salotti
Vice President, Operations, The Avoca Group |

