AQC Member Resources: There’s Still More for 2024!
There are exciting new Avoca Quality Consortium (AQC) resources to come in 2024. We’ll go back to basics with support throughout the study lifecycle, development of leading practices across multiple workstreams, and a continued focus on ICH E6 (R3) preparation.
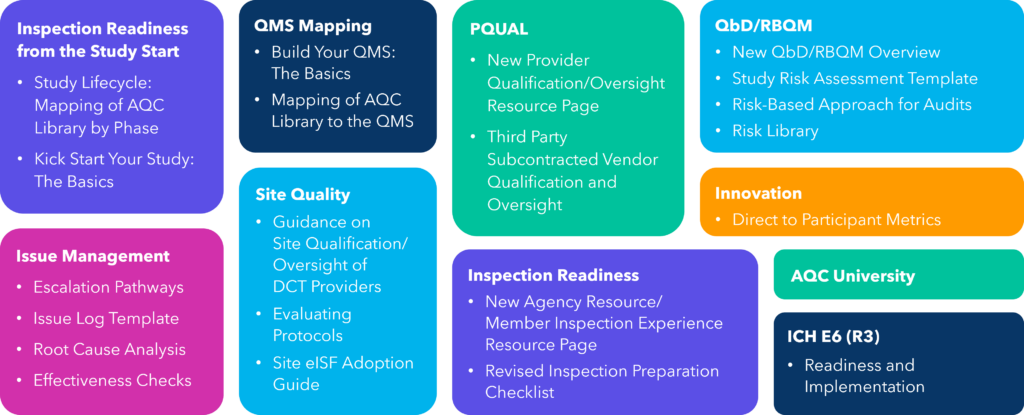
With the upcoming AQC member resources, you will:
- Get inspection-ready from the start with mapping of AQC tools for each phase of the clinical trial lifecycle and see what is needed to kickstart your study.
- Build your quality management system (QMS) and streamline processes with mapping of AQC tools to the QMS.
- Gain access to a provider qualification (PQUAL) and oversight resource page, with a new leading practice for third-party subcontracted vendor qualification and oversight.
- Mitigate risk with a new Quality by Design/Risk-Based Quality Management (QbD/RBQM) overview with supporting resources and a new leading practice for risk-based approach for audits.
- Utilize new leading practices for effective issue management, including escalation pathways, issue log template, root cause analysis, and effectiveness checks.
- Focus on site quality with guidance on site qualification/oversight of decentralized clinical trial (DCT) providers, protocol evaluation, and electronic investigator site file (eISF) adoption.
- Elevate inspection readiness, boost innovation adoption, leverage AQC University courses, and plan for ICH E6 (R3) readiness and implementation.
AQC members can head over to the Knowledge Center to access the library of 1,500+ tools, templates, guidelines, metrics, and more to support and improve clinical trial processes. Stay tuned as great new additions are released.
Contact us today to get involved! ›
// ABOUT THE AQC //
Founded in 2011, WCG’s Avoca Quality Consortium is a life sciences collaborative comprised of over 200 clinical research companies, including sponsors, CROs, sites, and clinical service providers. AQC members benefit from ready-access to industry resources for every stage of the clinical trial process and from in-person and online networking and collaboration opportunities.
|
