Ensuring Compliance with Standard Operating Procedures (SOPs)
Written and submitted by Craig Morgan, Head of Marketing, Study Startup, Oracle Health Sciences – an AQC Member company.
Standard Operating Procedures (SOPs) rarely grab the spotlight like transformational technologies or newer strategies, such as risk-based monitoring, quality by design, or decentralized clinical trials. But SOPs deserve some recognition. With their coveted goal of improving quality and operational efficiency, SOPs have long been fundamental to many industries, and the clinical trials sector is no exception.
SOPs are one of the most useful systems in helping to improve the quality and efficiency of clinical trial execution. Regulations require that SOPs be utilized and that, ultimately, clear documentation exists that demonstrate the processes were followed. This can aptly be described as “writing down what you do and doing what is written down.”
SOPs are a set of instructions that standardize a procedure or specific function, and are an important way to help sponsors and Contract Research Organizations (CROs) follow Good Clinical Practice (GCP) guidelines, as published by the International Conference on Harmonization (ICH).
There is virtually no regulatory guidance on SOP system design, which has meant that companies have been forced to create individual SOPs, which limits collaborations and automation within the industry. Yet, failure to comply with them can result in violations during regulatory audits.
The importance of well-written SOPs cannot be overstated in clinical trials, specifically they:
- Manage compliance obligations: SOPs ensure compliance with all regulations; and that all clinical operations are following the written SOP.
- Create operational efficiency: SOPs ensure processes have been examined and optimized. They standardize common processes amongst all studies.
- Reduce learning curve/training of staff: SOPs are a lifeline to new employees, detailing how activities are required to be performed; they act as a resource to keep everyone on the same page at all times.
- Ensure business continuity: SOPs allow for continued operations in the event of staff turnover or unavailability. By referring to the SOP someone with the required role and appropriate training can handle an urgent task and do it correctly the first time.
- Quality Control: SOPs help reduce errors or variations. They improve the quality of the data collected, thereby improving the science of the study.
The benefits of SOPs are clear, they provide a level of formal accountability for team members, and they prevent noncompliance on a systemic level.
Adopting Industry Leading SOP Guidelines, Tools, and Templates
The Avoca Quality Consortium® (AQC) has created hundreds of leading-practice documents that help companies increase efficiency, improve quality, and mitigate risk in clinical trials. Specifically, Avoca’s Quality Management System Framework, dedicates an entire section to SOPs as an important component to having a holistic QMS. When companies adopt and apply SOPs a level of confidence and efficiency is felt across the organization.
Despite the benefits and importance of SOPs, a major limitation of SOPs is noncompliance or, worst still, avoidance.
In Fiscal Year 2018, the FDA Office of Bioresearch Monitoring issued 216 violations known as “483s”, which are issued when inspectors notify management of objectionable conditions. Specifically, failure to follow written procedures, conduct clinical trials in accordance with signed documents or SOPs, or failure to keep accurate records and establish and maintain SOPs appear frequently in Form 483 violations and Warning Letters issued by the FDA. These findings indicate that companies may not have procedures that support operational processes and employees lack training or access to the SOPs.
Digitalizing and Automating SOP Workflows
Fortunately, cloud-based technology is available, which can digitize and automate workflows according to how a particular SOP is to be followed.
The automated workflow operates by configuring settings in real-time to accommodate changes in country specific regulations and/or organizational SOPs. Authorized team members can view and manage existing configurations and then edit them to create the settings needed for tracking documents, submissions, and milestones. Figure 1 shows a step-by-step workflow for a Standard Operating Procedure to initiate a study in the United Kingdom.
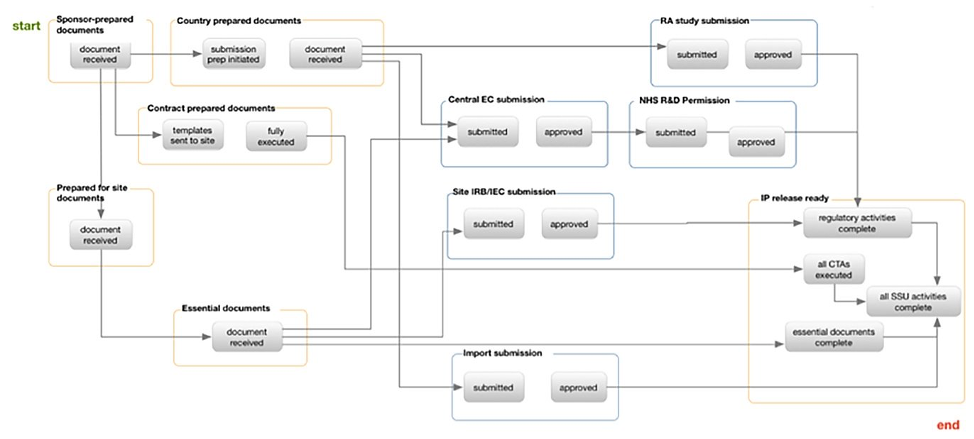
Fig 1. Step-by-Step Automated Workflow for starting a study in the United Kingdom
From an oversight perspective, the complexity of ensuring compliance in clinical trials is self-evident: How are all country specific documents tracked? Where are we in the process? Where are the bottlenecks and inefficiencies? Are we in compliance with our institutional SOPs? Furthermore, ensuring that the most recent versions of these documents are used can be challenging, if not a daunting task, especially if there are multiple versions and amendments across multiple countries.
With the advent of intelligent document routing technology, stakeholders have the ability to support country-specific document regulatory workflows. This functionality breathes life into SOP compliance and adherence to clinical timelines and budgets while improving quality and boosting operational efficiencies.
