Patient perspectives on clinical trial participation and engagement
Everyone is talking about patient engagement. The informed, empowered patients of today want to play an active role in the selection and management of their medical care and are not afraid to demand it. To understand this mindset better, we surveyed nearly 600 patients who shared their opinions on clinical trial participation: how they feel about the “quality” and value of clinical research, their confidence level in the clinical trial process, the information they are provided and why they would or would not choose to participate in a clinical trial.
Here are some interesting insights from Avoca’s 2016 Patient Survey.
- Forty-five percent of survey respondents had participated in a clinical trial.
- Among those respondents who had participated in a clinical trial, the “ability to contribute to science” was cited as the most frequent reason for participation.
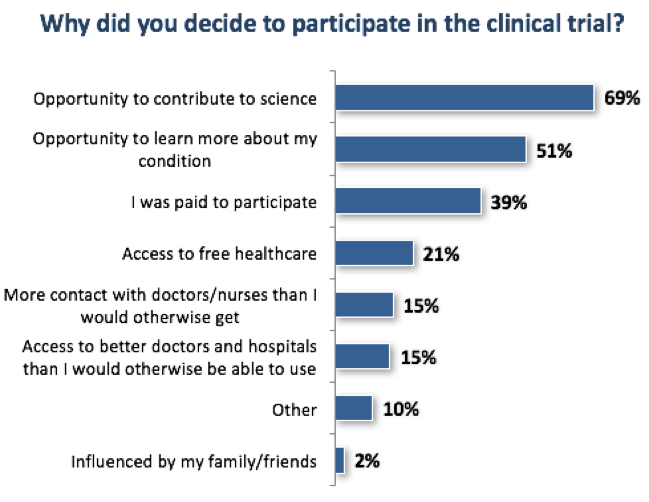
The top reasons provided for clinical trial participation did not differ significantly based on the type and impact of medical condition.
- Clinical trials are a great platform to engage patients in their health care. Two thirds of those participating in clinical trials reported feeling more educated and involved in their overall health as a result of their participation.
- Most of those surveyed had at least a fair level of confidence that trial participants are fully and honestly informed. Participants were most likely to be confident in information provided about the right to withdraw, procedures, and possible benefits, and were most skeptical about information relating to treatment alternatives, risks and side effects, and doctors’ motivations.
- Like with the confidence in information provided, most participants had at least a fair level of confidence in the compliance, caring, and ethics of the site study teams and patients involved in clinical trials; however, many were skeptical about the honesty of pharmaceutical companies and about the extent to which they cared about study patients.
- Sixty-seven percent of survey participants would recommend clinical trial participation to a loved one or friend. Among those who would recommend participation, responses carried themes of consideration of risks vs. benefits, of the institutions at which the studies are being performed, and of a wish to “do good” for society. Among those who would not, responses generally reflected a distrust of the clinical research industry, or concerns about the possibility of receiving ineffective treatment or placebo.
In the participants’ own words, here are some of the gaps that need to be bridged by the industry to get more patients to participate and recommend clinical trials.
Interaction with patients for feedback about study design and operations
“They don’t interact with participants to get feedback on what was good or bad about the study.”
Fair compensation, and caring, respectful, and equitable treatment as participants in the research process
“While the protocol is patient-centered, the back-up and bedside manner do not always work.”
“Care about patients as people? Ha! Then why are we called ‘subjects’?? And why aren’t we compensated much better and sooner in the study? ……….Why are the visits always so inconvenient? Why aren’t there evening and weekend hours? Why aren’t staff trained in interpersonal communication, especially the chief investigating physician?”
Transparency across the board
“The medical and pharmaceutical industries’ main priority is money! When money is the most important thing, people are not told complete information. It’s a basic conflict of interest!”
“Re. doctors’ motivations, I don’t remember this particular aspect of either trial we participated in, or the various other trials I considered, ever having been mentioned.”
“I asked for feedback after the study I did participate in and never received it. I know of no-one who trusts pharmaceutical companies.”
Overall, the results from this study indicate that as an industry, we can engage more effectively with patients on clinical trials, impacting their perceptions of clinical trial participation, and their experience.
At the Avoca Quality Consortium (AQC), we are not only sharing these results through multiple platforms, but are working diligently to weave these learnings into the creation of a “Patient Engagement Playbook” that will enable our Members to easily incorporate the patient voice into multiple stages of the clinical trial process.
To download a copy of the complete results, click here. To learn more about our patient engagement initiatives and/or AQC membership, please contact us.
